
Note that this is the WRONG structure at a neutral pH. Here is an example of deprotonating a protonated R group (glutamic acid).
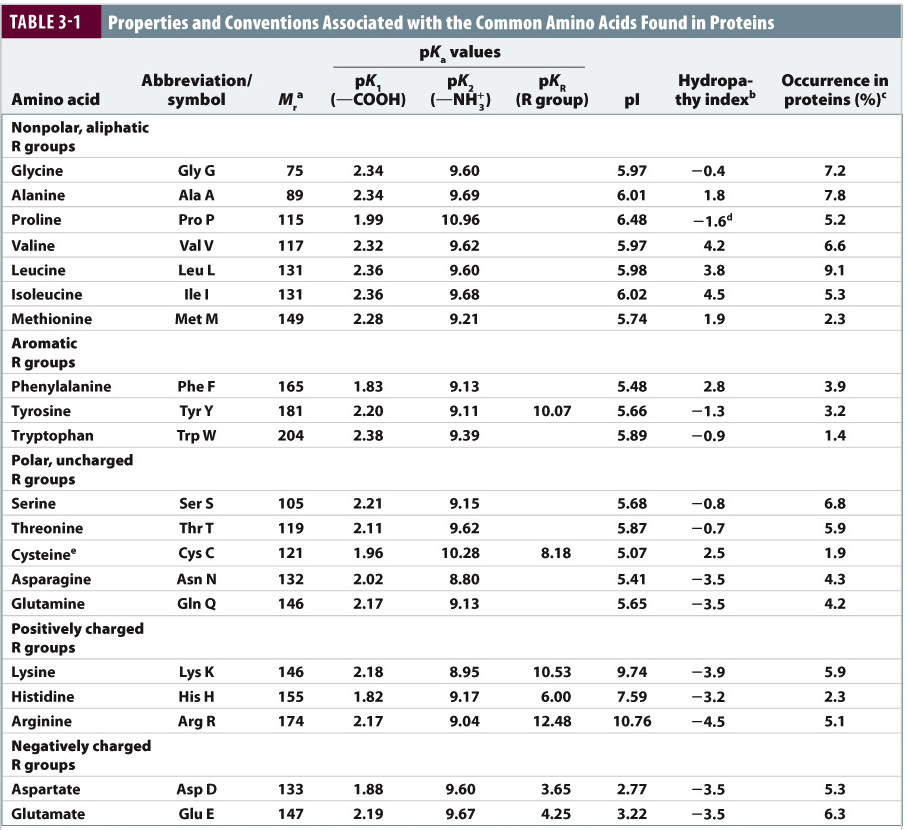
pI is half way between these values: pI (9.87 + 10.35) 10.20. The zwitterionic form is the third form drawn overleaf, occurring between pH 9.87 and 10.35. If you start at a high pH and decrease it, read the structures from right to left. How do you calculate the isoelectric point of a dipeptide The dipeptide has 2 amine and one carboxylic acid group left after the formation of the amide bonds. Here is the structures at increasing pHs for a generic amino acid. The #pK_b# values for amino groups are lower than that of carboxyl groups, so the amino groups will be protonated before the carboxyl groups. The exact opposite would happen for protonation of amino acids. Use the following link to find a list of the #pK_a# values for all the amino acids. The atom with the lowest #pK_a# will be deprotonated. As pH increases, it will be deprotonated before the ammonium group.įor the amino acids with protonated R groups, you need to pay attention to their #pK_a# values. The #pK_a# of the carboxylic acid is always lower than that of the ammonium group. Look at the #pK_a# values of the ammonium and carboxyl groups. This is due to ammonium (amino) groups being less acidic than carboxylic acids. Amphoteric, dipolar species are called zwitterions. Amino acids are amphoteric, meaning they can act like an acid and base. Given that B A B A and X A + X B 1, we can derive these equations: To state it another way, 99.9981 of the carboxyl groups are in the deprotonated (negative) state and 99.3872 of the amino groups are in the protonated (positive) state. Notice something weird? The amino group is protonated but the carboxyl is not.

Let's start by looking at the generic structure of an amino acid. The pH of an amino acid affects which atoms protonate and deprotonate.


 0 kommentar(er)
0 kommentar(er)
